La maschera per terapia LED di Wakelife riceve ufficialmente la FDA 510(k) Spazio
Siamo orgogliosi di annunciare una pietra miliare fondamentale nella nostra storia di innovazione. La maschera per terapia della luce LED di punta di Wakelife è stata ricevuta con successo 510(k) autorizzazione dagli Stati Uniti. Food and Drug Administration (FDA).
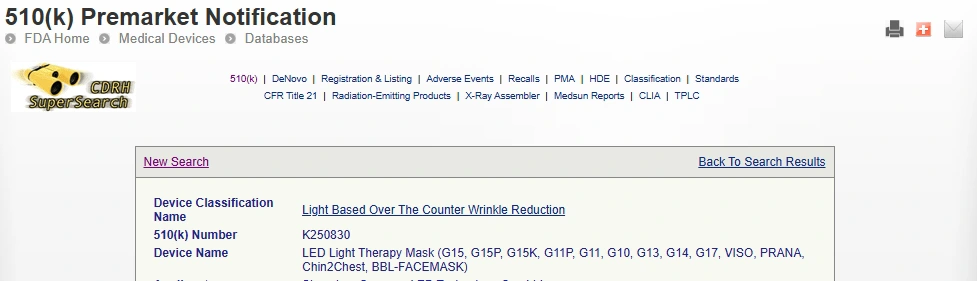
Questo risultato è pubblicamente verificabile sul funzionario banca dati della FDA sotto il numero di presentazione K250830.
Questo è più di un semplice certificato; è una testimonianza del nostro costante impegno per la sicurezza dei prodotti, efficacia, ed eccellenza normativa. Per i nostri partner: i marchi di prodotti per la cura della pelle, rivenditori, e i distributori che serviamo: questa autorizzazione rappresenta un'opportunità per offrire con sicurezza un prodotto di prima qualità, dispositivo riconosciuto dal punto di vista medico da commercializzare.
Come cercare la FDA di una maschera facciale a LED 510(K) rapporto
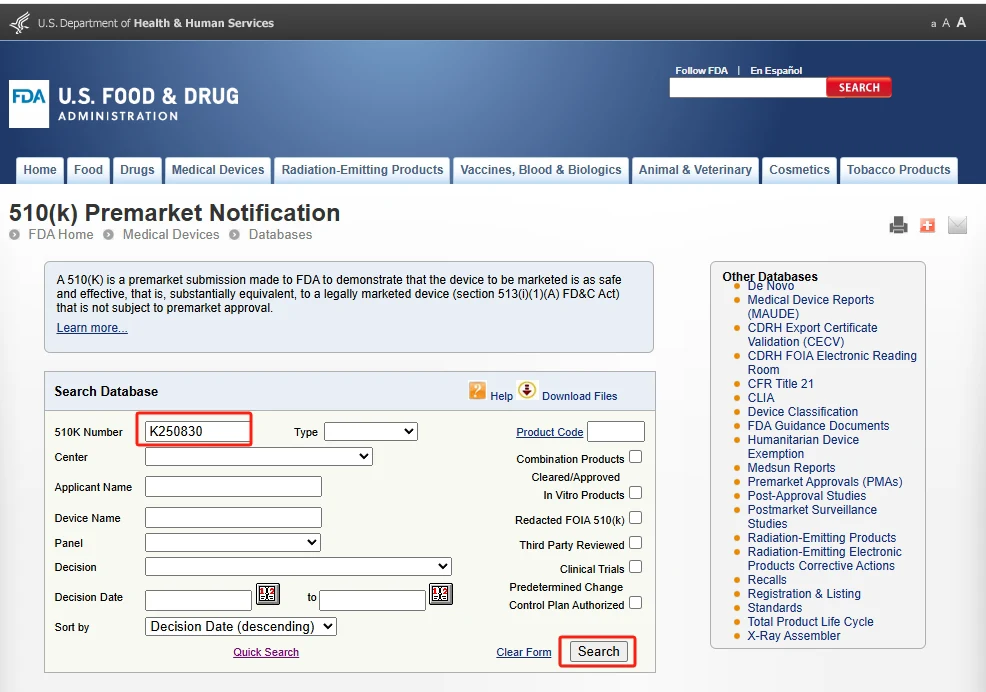
Apri il sito web della FDA per 510(K) pagina di ricerca: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm.
Inserisci il numero K250830 e fare clic ricerca per visualizzare la FDA completa 510(K) rapporto.
Perché la FDA 510(k) è il Gold Standard nella tecnologia della bellezza
Per chi non ha familiarità con il panorama normativo, il termine “FDA 510(k) spazio” potrebbe sembrare complesso. In termini semplici, è il percorso primario per i dispositivi medici di Classe II, che comportano un livello di rischio moderato, essere commercializzato legalmente negli Stati Uniti. È un metodo rigoroso, processo impegnativo che separa i dispositivi veramente controllati dal vasto mercato dei gadget non verificati.
Il principio fondamentale di a 510(k) la sottomissione sta dimostrando Equivalenza sostanziale (SE). Ciò ci impone di fornire alla FDA ampie prove scientifiche e tecniche che dimostrino che il nostro dispositivo è sicuro ed efficace quanto un dispositivo commercializzato legalmente “dispositivo predicativo.”
Per raggiungere questo obiettivo, la FDA esamina ogni aspetto del prodotto, tra cui:
Ingegneria & Progetto: Schemi e specifiche dettagliate.
Test di biocompatibilità: Garantire che tutti i materiali a contatto con la pelle siano atossici e non irritanti.
Dati sulle prestazioni: Test rigorosi per l'emissione di energia luminosa, precisione della lunghezza d'onda, sicurezza elettrica, e compatibilità elettromagnetica (EMC).
Gestione completa del rischio: Un’analisi completa e la mitigazione di eventuali rischi potenziali.
Etichettatura & Manuali utente: Garantire che tutte le dichiarazioni siano accurate e che le istruzioni siano cristalline per l'utente finale.
Questa non è una semplice registrazione. Si tratta di una revisione esaustiva che conferma che un dispositivo soddisfa i più elevati standard di prestazioni e sicurezza di livello medico.
Il tuo partner strategico per la crescita
Ricevere la FDA 510(k) lo sdoganamento è un momento decisivo per Wakelife, ma soprattutto, è un adempimento diretto della nostra promessa nei tuoi confronti, i nostri partner. Rafforza il nostro ruolo non solo come produttore, ma come alleato strategico dedicato a fornire conformità a livello globale, sicuro, ed efficaci soluzioni di bellezza che guidano la crescita del mercato.
Costruiamo dispositivi che costruiscono marchi. Ora, lo facciamo con il sostegno ufficiale dell’organismo di regolamentazione più rispettato al mondo.
Pronto a elevare il tuo marchio con l'approvazione della FDA, prodotto eroe collaudato sul mercato?
[Contatta i nostri specialisti oggi stesso per richiedere un campione e discutere il tuo piano di partnership esclusivo.]
Già partner di Wakelife?
[Contatta il tuo account manager per ricevere risorse di marketing aggiornate e sfruttare queste incredibili novità nei tuoi canali di vendita.]




